A flagship biomedical research institute for the Gold Coast Health and Knowledge Precinct, the Institute for Biomedicine and Glycomics is regarded globally as key infrastructure in the commercial pipeline for drug and vaccine development.
With more than 200 researchers, the institute is a world leader in the discovery of next-generation drugs, vaccines and diagnostics for diseases of global impact such as cancer, infectious diseases and neuro-degenerative disorders.
The institute commercialises its technologies through partnerships with a range of domestic and international biotechnology companies (including LimmaTech Biologics, INOVIQ, Olymvax, Lilly and Sirtex) and has a number of technologies in the commercial pipeline or available for investment.


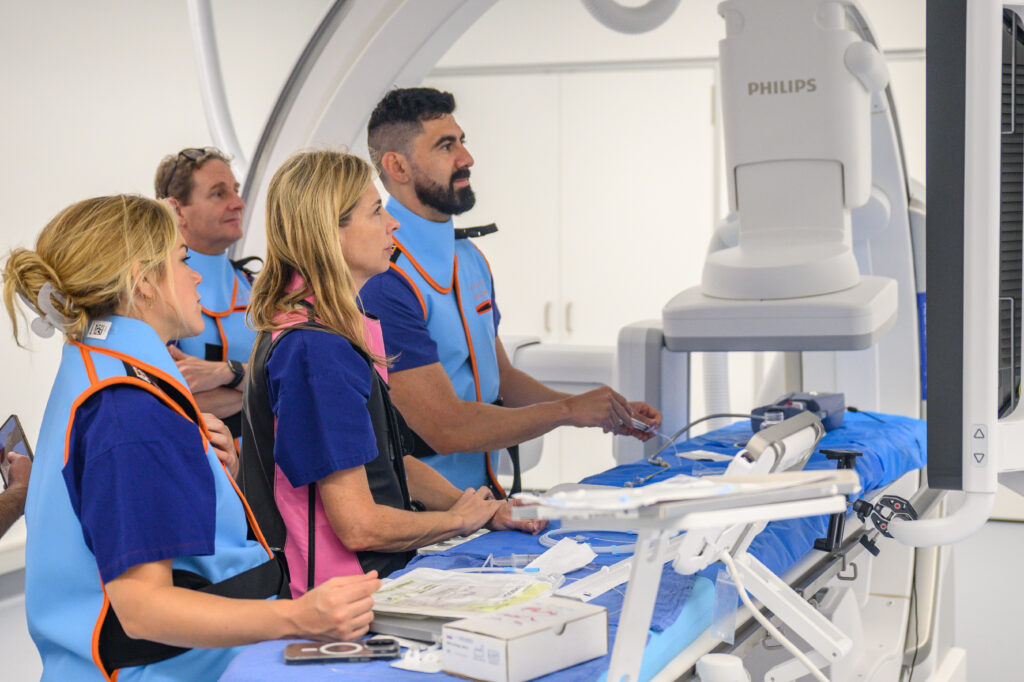




 Griffith University’s Institute for Biomedicine and Glycomics and Clinical Trial Unit are central to the university’s partnership in the
Griffith University’s Institute for Biomedicine and Glycomics and Clinical Trial Unit are central to the university’s partnership in the 


