
The Gold Coast Health and Knowledge Precinct is drawing national and international attention as a clinical trial hot spot, with a senior Austrade representative outlining the growing opportunities for the Precinct in the global clinical trial landscape.
Griffith University’s Clinical Trial Unit (CTU), together with the Gold Coast Health and Hospital Service, are fast building the GCHKP’s clinical trial capacity through a collaborative approach that brings researchers and clinicians together with industry partners to build business opportunities and best practice, supported by Australia’s cost-advantage and reputation.
Collaboration was formalised through an MOU, signed in November 2017, and continues to build with more than 100 registered trials either underway or completed.
Speaking at the inaugural ‘lunch and learn’ event, Austrade’s A/g Manager International Health, Mr Abdul Ekram, outlined the cost advantage of clinical trials in Australia, with early-phase trials 60% cheaper than they are in the United States, after tax incentives.
1,300 clinical trails are conducted in Australia each year, 63% involving industry, with the US sponsoring around 6,000 trials a year, 260 of which are conducted in Australia – there is a real growth opportunity
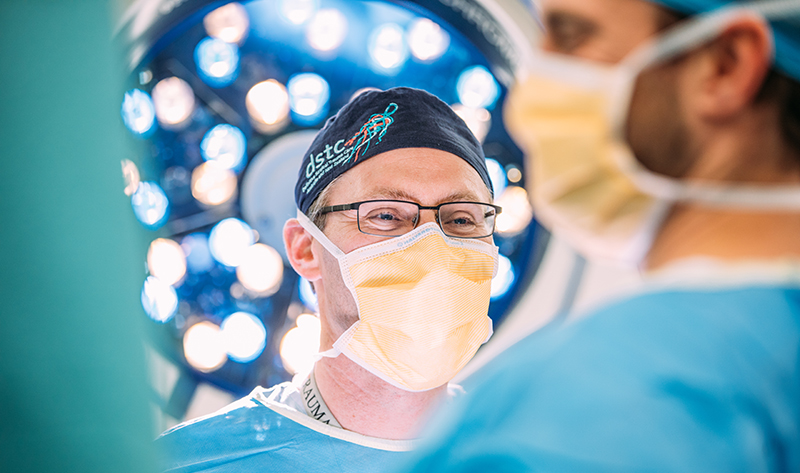
Trials range from Phase I to IV, including a second stage (1b) trial of the novel malaria vaccine PlasProtecT®, developed by the Institute for Glycomics’s Professor Michael Good AO and Senior Research Fellow Danielle Stanisic through extensive research since 2010; to a trauma study investigating whether early cooling of patients with severe traumatic brain injury produces better outcomes, led by the GCUH’s Director of Trauma and Griffith University Professor of Traumotology, Dr Martin Wullschleger.
Many trials are national and international multi-centre studies, including as part of Clinical Trials Networks such as the Australasian Kidney Trials Network, Australasian Stroke Trials Network and the National Trauma Research Institute, and conducted with leading global pharmaceutical and medtech companies, including Stryker, Medtronic, Paradigm Biopharmaceuticals and more.
Trials range across pharmaceutical studies for new and existing drugs, medical devices and surgical procedures to cancer treatment and a full range of medical specialty areas including haemotology, oncology, cardiology, endocrinology, interventional neuroradiology (led by global expert and GCUH specialist Dr Hal Rice), neurology, emergency medicine, surgery, trauma, obstetrics and gynaecology, paediatrics, allied health and complimentary medicine.
A full-list of Gold Coast Health registered trials is available here.
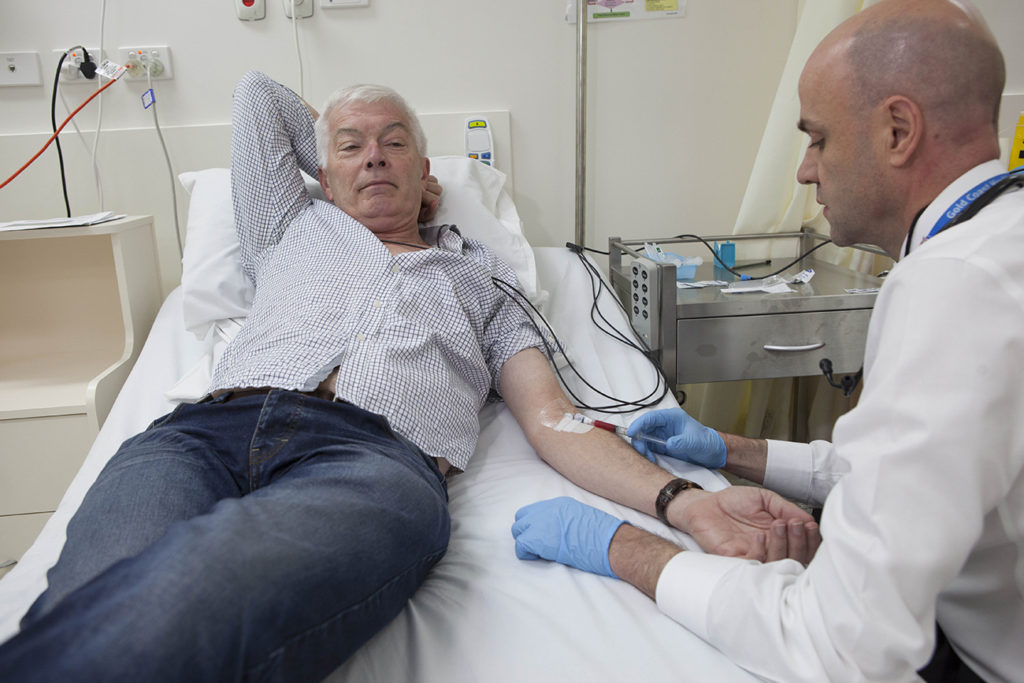
Griffith University’s Clinical Trial Unit (CTU), located in the Griffith Health Centre on the Gold Coast campus adjacent to Gold Coast University Hospital, offers purpose-built, GCP aligned facilities for Phase I–IV clinical trials.
As a Core Research Facility of the University, the unit supports staff and collaborators to conduct a wide range of investigator initiated trials. It also provides professional trial coordination services to external clients such as the pharmaceutical, biotech, nutraceutical and complementary medicine industries, as well as Clinical Research Organisations.
The CTU has successfully conducted trials in:
- Musculoskeletal Diseases
- Infectious Diseases
- Nephrology and Renal Disease
- Endocrinology and Metabolic Disorders
- Neurological Disorders
- Device trials
- Pharmacokinetic studies
A full list of current trials managed by the CTU is available here and donate or learn more about the Malaria Vaccine trial via the project website.
Gold Coast Private Hospital is also involved in a number of clinical trials in specialist areas, including cardiology, oncology and neurosurgery.
