We know that great research changes lives, but only when it reaches the people who need it. That’s why our precinct enables practical research outcomes through strong partnerships, shared infrastructure, and a focus on commercialisation and implementation.
Knowledge + Collaboration =
Real Impact





Why research here?
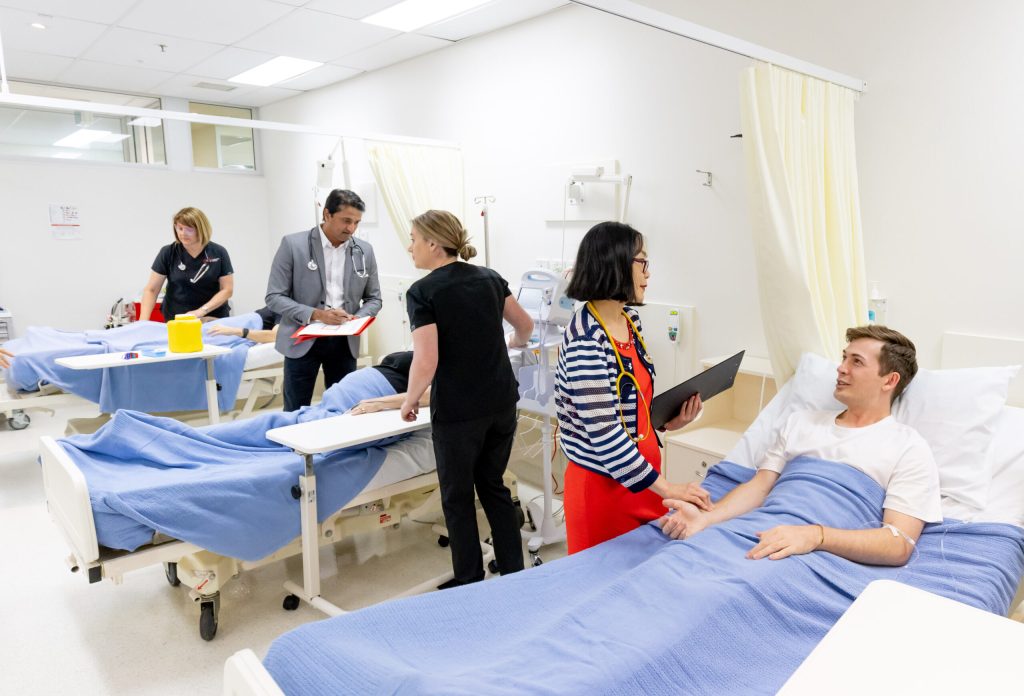
The GCHKP is built for collaboration and grounded in purpose. As a researcher in the precinct, you’ll be part of an active community accelerating breakthroughs across health, science, engineering and technology.
Here’s what you’ll find:
- Embedded partnerships with Griffith University and Gold Coast Health
- Access to clinical environments, trial units, hospital data and ethics pathways
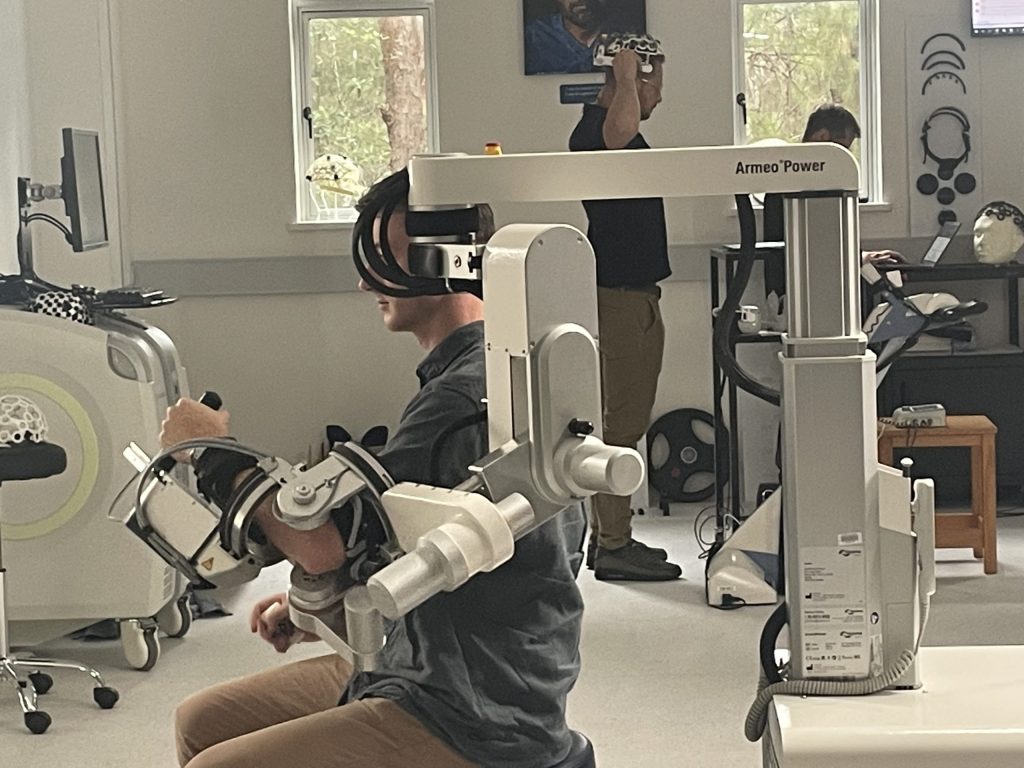
- State-of-the-art research infrastructure, including the Institute for Glycomics, ADaPT, PRECISE, and the upcoming HATRIC facility
- Opportunities to co-design and commercialise through startup programs and translational platforms
- Grant-ready collaborations with academic, industry and government partners
- A precinct-wide focus on social impact, applied science and community benefit
Whether you’re leading a research centre, starting a PhD, or commercialising an innovation, our ecosystem is designed to help you go further.
Ready to partner, pilot or scale
Your research?
We’re here for it!









